Corbevax becomes third Covid-19 vaccine to be approved for children in India
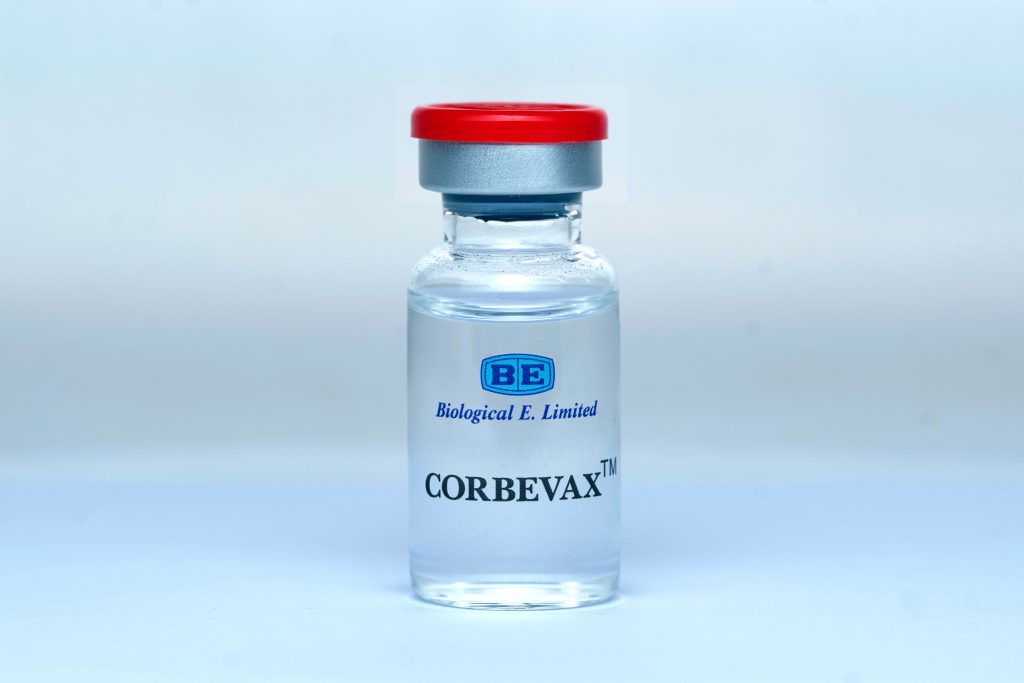

NEW DELHI: Biological E. Limited (BE), one of the world’s largest vaccine manufacturers, has today announced that its CORBEVAXTM vaccine, which is India’s first indigenously developed Receptor Binding Domain (RBD) Protein sub-unit vaccine against COVID-19, has received emergency use authorisation (EUA) from India’s drug regulator for the 12 to 18-year age group.
As per the report, Cadila Healthcare’s ZyCoV-D and Bharat Biotech’s Covaxin are two vaccines that have already been approved in this category.
The Drugs Controller General of India (DCGI) has already approved CORBEVAXTM for restricted use in emergency situation among adults on December 28, 2019. BE received the approval for restricted use in an emergency situation in adolescents aged 12 to less than 18 years based on interim results (of the ongoing phase II/III clinical study).
Mahima Datla, Managing Director, Biological E. Limited, said, “We are pleased with this significant development, which helps extend the reach of our vaccine to the age group of 12 to 18 years in our country. We truly believe that with this approval, we are even more closer to finishing our global fight against the COVID-19 pandemic. Once fully vaccinated, children can resume their activities and educational pursuits in schools & colleges without any apprehension. We thank all the participants in the clinical trials, Biotechnology Industry Research Assistance Council (BIRAC) and Department of Biotechnology, Govt of India, Translational Health Science and Technology Institute (TSTHI) and the principal investigators and clinical site staff who have extended their support during the last several months.’’
Last September, BE received approval to conduct a Phase II/III clinical trial on CORBEVAXTM in children and adolescents aged 5 to 18 years. Based on the no-objection certificate, BE initiated the clinical study in October 2021 and evaluated the available safety and immunogenicity results of the ongoing phase II/III study, which indicated that the vaccine is safe and immunogenic.
The CORBEVAXTM vaccine is administered through intramuscular route with two doses scheduled 28 days apart and is stored at 2 to 8 degrees’ Celsius temperature and presented as 0.5 ml (single dose) and 5 ml (10 doses) vial and 10 mL (20 doses) vial pack
BE conducted phase I/II, II/III clinical trials of its CORBEVAXTM vaccine for adults in the country. In addition, it conducted a Phase III active comparison clinical trial to evaluate superiority over Covishield vaccine.