Apollo, Dr Reddy’s announce Covid-19 vaccination programme with Sputnik V
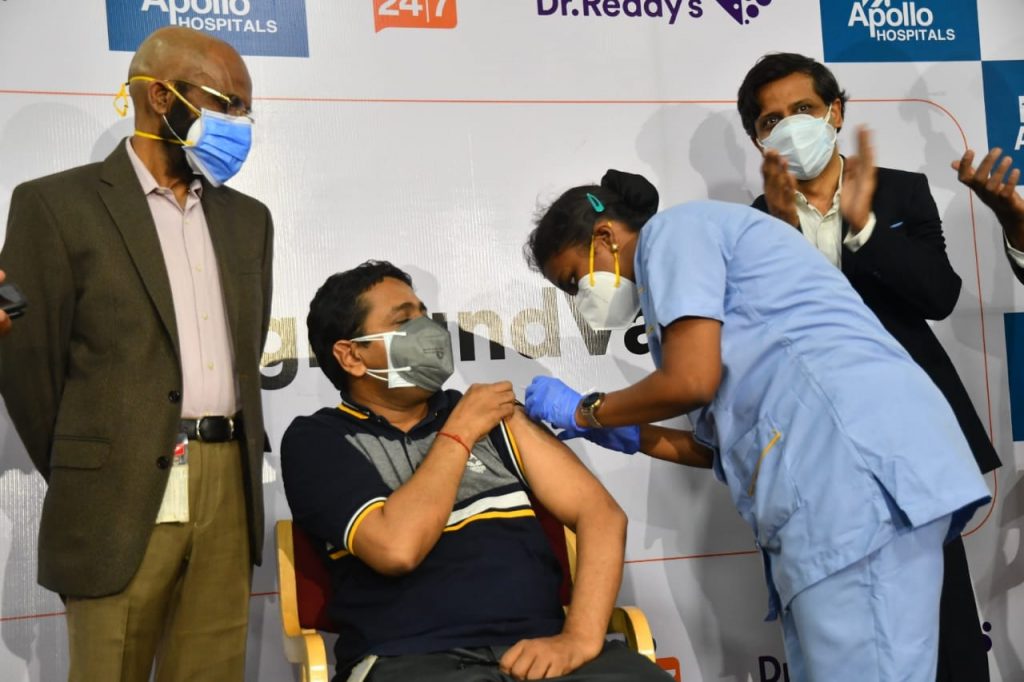

NEW DELHI: Apollo Hospitals and Dr Reddy’s Laboratories on Monday said they are collaborating to initiate a COVID-19 vaccination programme with Sputnik V.
The first phase of the programme will kick off with vaccinations in Hyderabad on Monday and in Visakhapatnam on Tuesday (May 18) at Apollo facilities.
The vaccinations would follow the SOPs as recommended by the government including registration on CoWIN.
“This pilot phase will allow Dr. Reddy’s and Apollo to test the arrangements and cold chain logistics and prepare for the launch. We are confident that with the Sputnik V vaccine, we will be able to make a significant contribution to ease availability and access to COVID vaccines to the community at large,” Apollo Hospitals President Hospitals Division K Hari Prasad said in a statement.
With the opening up of the vaccination programme for the private sector, the healthcare major has intensified efforts to accelerate the rate of vaccination through opening vaccination centres across its hospital network, he added.
“We are also in discussions with corporates to undertake vaccination on their premises. We are currently administering COVID vaccine at 60 locations across the country including Apollo Hospitals, Apollo Spectra hospitals and Apollo Clinics,” Prasad said.
Dr Reddy’s Laboratories CEO Branded Markets (India & Emerging Markets) MV Ramana noted that the two entities were working to scale up the pilot and take the vaccine to other cities with an aim to inoculate as many Indians as possible.
The Sputnik V vaccines for the pilot programme would be supplied by Dr Reddy’s from the first batch of 1.5 lakh doses imported so far.
After Hyderabad and Visakhapatnam, the pilot programme will be extended to Delhi, Mumbai, Bengaluru, Ahmedabad, Chennai, Kolkata, and Pune.
In August 2020, Russia became the world’s first country to register a coronavirus vaccine, dubbed as Sputnik V.
Following that, in September, Dr Reddy’s Laboratories and Russian Direct Investment Fund (RDIF) entered into a partnership to conduct clinical trials of Sputnik V, which is developed by the Gamaleya National Research Institute of Epidemiology and Microbiology.
Dr Reddy’s Laboratories has received approval from the Indian drug regulator for restricted emergency use of Sputnik V.
Source: Press Trust of India