Dr Reddy’s expects Covid-19 vaccine to get nod from India in next few weeks
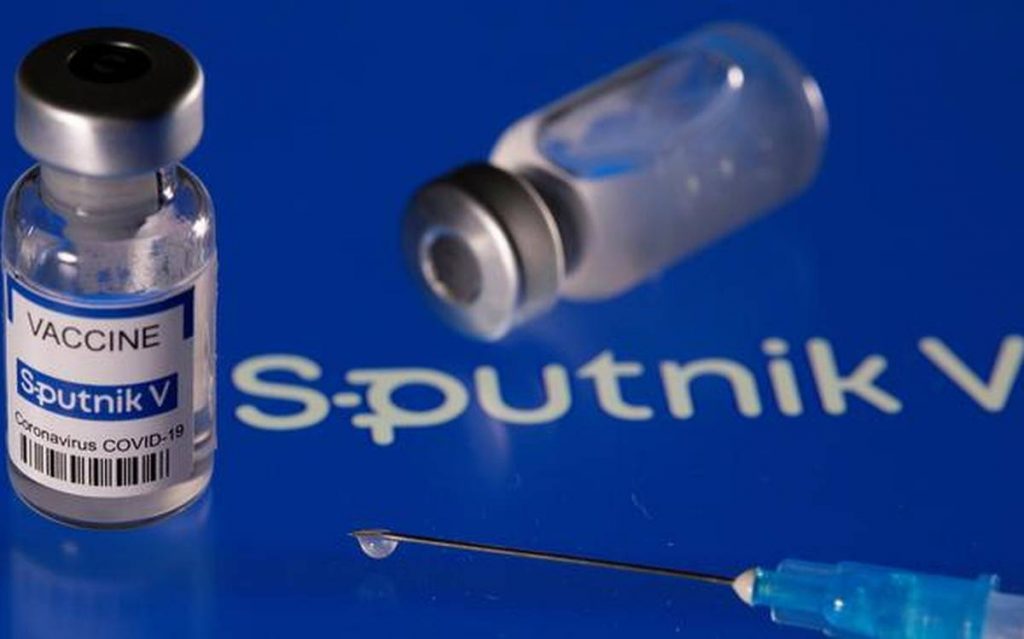

NEW DELHI: Pharma major Dr Reddy’s Laboratories expects the Russian coronavirus vaccine Sputnik V to get approval from the Indian regulator in the next few weeks, a company official has said.
“We expect to get the approval in the next few weeks. It is a two dose vaccine. You take the first dose on day zero and the second one on day 21. The peak immunity develops somewhere between day 28 to day 42. So, it is a two-dose vaccine and we expect it to be available in the next few weeks,” Deepak Sapra, the companys CEO, APIs and Services, said.
Sapra, who was speaking in a webinar on Sunday evening, was asked as to how soon Sputnik would be available and whether it is a one dose or two doses vaccine.
Dr Reddy’s has partnered with the Russia Direct Investment Fund (RDIF) to bring the Sputnik V vaccine to India and other countries, he said.
Observing that trials of the vaccine have been conducted in Russia, India, the UAE and others, he said the vaccine demonstrated an efficacy of 91.6 per cent as published in reputed journal Lancet.
The data on trials is currently with the Indian regulator and the company expects it to get approved in the next few weeks, he said.
“Now, what we have done in India, in addition to all this, we also conducted trials in India and evaluated the vaccine on the Indian population for both safety as well as for immunogenicity. This data is currently with the Indian regulator and we expect it to get approved in the next few weeks,” he said.
The webinar on “India’s vaccination journey and the second wave of COVID-19′ was conducted by the All India Professional Congress (AIPC), Telangana.
Former minister J Geeta Reddy, a medical doctor, moderated the event.
Mahima Datla, Managing Director of Biological E, and Nageshwar Reddy, Chairman of AIG Hospitals, spoke on various important issues concerning COVID-19 surge and the vaccines.
Source: Press Trust of India